The Advantages of Onshore Progressive Stamping for MedTech OEMs

The Power of a Unified, Customer-Centric MedTech Design and Manufacturing Partner

Your Medical Device’s User Interface Specification Is More Powerful Than You Think

Risk Management in Action: Harms and Probabilities of Occurrence and Severity for Your Medical Device
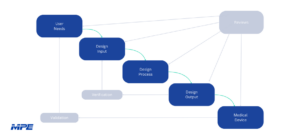
Does Your Medical Device Need Design Controls? Here’s What You Need to Know.

Breaking Down the Verification and Validation Processes for Medical Devices

4 Services to Meet Any Medical Device Development Challenge Head-On
How to Achieve IEC 60601-1 Without Slowing Down Your Time to Market

Addressing Manufacturing Labor Shortages by Investing in Students