Seamless development begins with a unified process.
Ready your medical devices and capital medical equipment for transfer and distribution with purposeful, scalable manufacturing operations. MPE Inc.’s integrated processes allow for a smooth transition from validation to launch with the support of in-house bill of materials (BOM) management and production capabilities.
Reduce complexities on the path to regulatory approval.
Through our unified processes, MPE Inc. conducts careful documentation at each step, securing an efficient product approval process. Beyond that, we ensure your product achieves the highest quality standards and is tested and validated as required.
Throughout medical device manufacturing, our commercialization platform provides:
- Component manufacturing
- Manufacturing readiness
- Quality and regulatory compliance
- Finished assembly
- Engineering and production
- Transfer to manufacturing
Our manufacturing capabilities provide assurance that you won’t be tripped up, whether you need 20 units or 200,000. Our low- to mid-volume capabilities offer flexibility as you need to scale. In addition, our control planes are designed to ensure differentiation, compliance, and market readiness are a clear focus at every phase on your path to commercialization.
Achieving predictability in a shifting market.
Working with MPE Inc. means setting out on a predictable path to market. We ease you over speed bumps, getting your product to market quickly, efficiently, and at a lower cost.
To see your device succeed, we see both the broad view and the tiniest details — all so your product provides value that users simply cannot ignore.
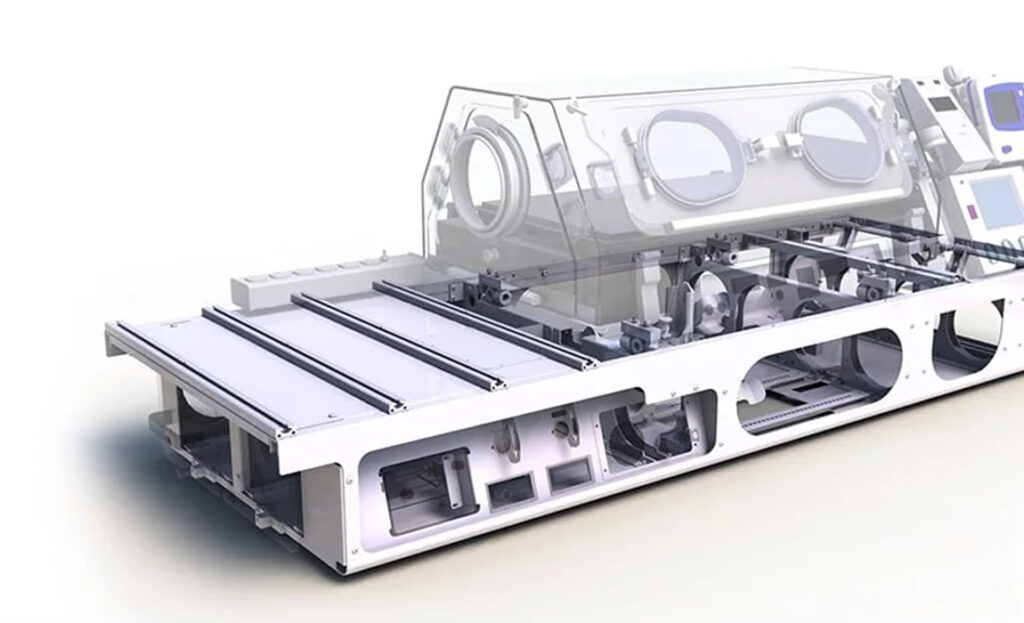
A new manufacturing process helped guarantee reliability and durability under the extreme conditions.
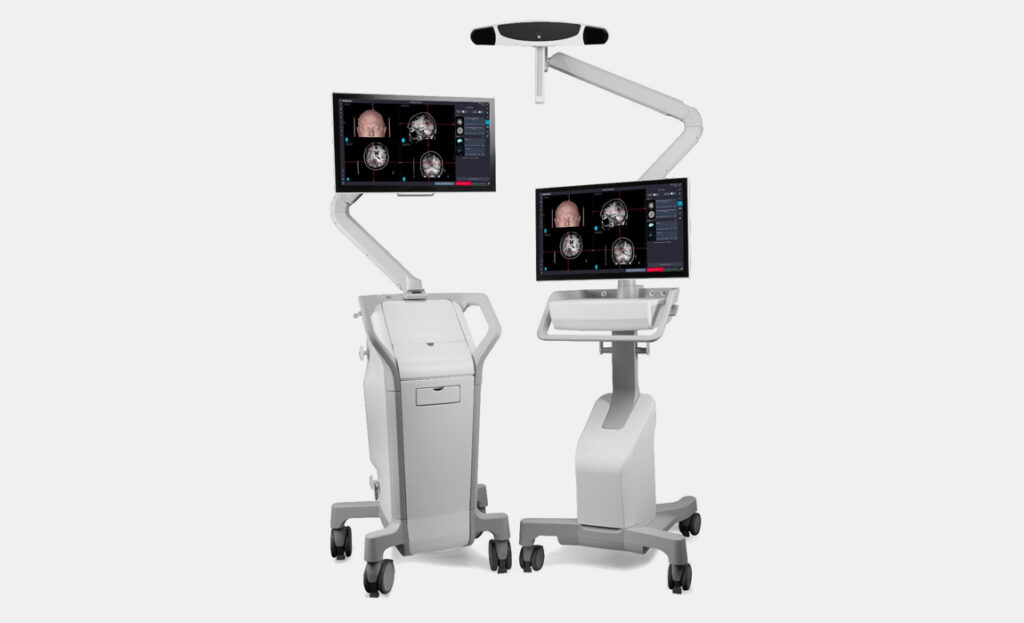
A newly developed, user-focused, two-piece surgical navigation system that simplifies transportation while requiring a minimal footprint within operating rooms.